The Different Types of Fuel Cell
10 min read
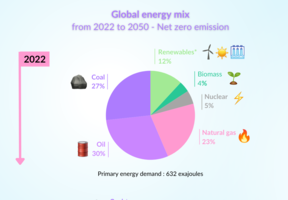
cells convert energy from a fuel into via an electrochemical reaction. The most commonly used fuels are natural gas and , which is discussed here.

© AFP PHOTO / SEBATIEN BOZON - An engineer demonstrates a PEMFC at Université de Belfort-Montbéliard in France.
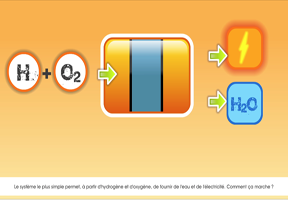
A using hydrogen consists of two electrodes:
- One positive, known as the anode, containing hydrogen.
- One negative, known as the cathode, containing oxygen.
An electrolyte — a solid or liquid able to control the flow of electrons — is sandwiched between the two. It is usually a polymer containing a platinum catalyst.
The hydrogen molecules separate in the anode: the ions are diffused in the electrolyte, while the electrons circulate in an external circuit, generating usable direct current.
At the cathode, electrons from the external circuit, the hydrogen ions (protons) and the oxygen combine to form water and that can be recovered.
Ultimately, with hydrogen on one side and oxygen on the other, the fuel cell generates both electricity and heat. The only byproduct of hydrogen combustion is water vapor.
Two Main Pathways
There are many fuel cell technologies, but the two main ones have already found significant industrial applications: proton exchange membrane fuel cells (PEMFC) and solid oxide fuel cells (SOFC):
- Solid oxide fuel cell (SOFC) technology is well suited to (combined heat and ). These fuel cells have an yttria-stabilized zirconia electrolyte. Their operating temperature of 800 °C enables any fuel containing hydrogen to be used, thanks to internal reforming processes that eliminate the need for pure hydrogen. This means that they could even run on municipal natural gas. SOFCs offer excellent power conversion efficiency — between 40 and 70% — as well as good thermal efficiency. The heat produced is reused in part to operate the fuel cell, while the very hot residual heat can be easily recovered. SOFCs are very heavy, have a low tolerance for vibrations and are easily disrupted by frequent stops. They are therefore intended for stationary applications, such as power generation for apartment buildings.
- Proton exchange membrane fuel cells (PEMFC) are compact and operate at low temperatures (80 °C) with a polymer electrolyte. They mainly provide electricity, with an average efficiency of 40 to 60%. This “all-terrain” technology can be used for both portable and stationary applications. R&D continues, especially to reduce its cost, which is high because platinum is used for both the anode and the cathode. PEMFCs are the only fuel cells suitable for transportation applications. They can also be used for niche stationary applications, such as supplying power to isolated sites or emergency power generation.
Among the other technologies, direct fuel cells (DMFC) and direct ethanol fuel cells (DEFC) can easily be miniaturized. They use the hydrogen contained in the respective alcohol molecules directly. Their main applications are mobile devices, such as cell phones and laptops.
Lifespan
Lifespan — the period of time during which the fuel cell performs optimally — is an important factor for industrial applications. It varies considerably depending on the type of fuel cell and the way it is used. For stationary fuel cells, the average lifespan today is between 30,000 and 40,000 hours. For fuel cells designed for cars, manufacturers have obtained lifespans of around 5,000 hours.
It should also be noted that fuel cells are very quiet — 40 to 50 decibels at a distance of a meter, which is lower than the volume of a normal conversation.