How Do Lithium-Ion Batteries Work?
5 min read
First invented more than 30 years ago, lithium-ion or Li-ion batteries have become a ubiquitous part of our daily lives, from the tiny versions in cell phones to the tenfold stacks used to electric cars. They are the subject of intense research efforts all over the world as a solution to the pressing challenge of storage.

© TOSHIFUMI KITAMURA / AFP - Hundreds of lithium-ion cells are grouped together to power electric or hybrid vehicles, such as the Toyota Prius pictured here.
How Batteries Work
Batteries are devices that convert chemical energy into electrical energy. Rechargeable batteries, also known as accumulators, can accept and store electric energy and release it when needed. This means they can be discharged and charged up again in a reversible process. Single-use electric batteries supply electricity to an external circuit until they run out of charge.
Batteries contain two electrodes immersed in an electrolyte – a conductive liquid or solid – and connected outside the electrolyte by a conductive wire. When discharging, the negative electrode (anode) releases electrons that travel along the wire and are absorbed by the positive electrode (cathode). This movement of electrons creates an electric current, which can then be converted to power a motor or electronic device. To balance out the exchange, positive ions travel through the electrolyte between the two electrodes. When the battery is charging up again with an external electricity source, this process is reversed.
Different Types of Rechargeable Batteries
Rechargeable batteries use combinations of materials that can easily and durably exchange electrons and positive ions. Internal combustion engine vehicles most often use lead-acid batteries, which contain a negative electrode made of lead, a positive electrode made of lead oxide, and an electrolyte consisting of sulfuric acid and water. Other materials used in batteries include nickel, cadmium, sodium and sulfur.
Scientists became particularly keen on lithium for batteries, since it is a very lightweight metal (the third element in the periodic table, after and helium). Lithium atoms can readily release one of their three electrons, creating positively charged Li+ ions. Manufacturers initially used lithium metal for the negative electrode, which emits electrons. However, they noticed that the repeated use and recharging cycles altered the metal. To avoid this, the cathodes are now often made from cobalt oxide and a small proportion of lithium, with a graphite anode. The electrolyte is made of lithium salts in a solvent, meaning that it contains a great many lithium ions. That is where the name “lithium-ion battery” comes from.
Lithium-Ion Cells
The core component of a lithium-ion battery is a cell that looks a bit like puff pastry, with an aluminum plate to collect the current, followed by the cathode, electrolyte, anode, and finally a copper plate (see diagram).
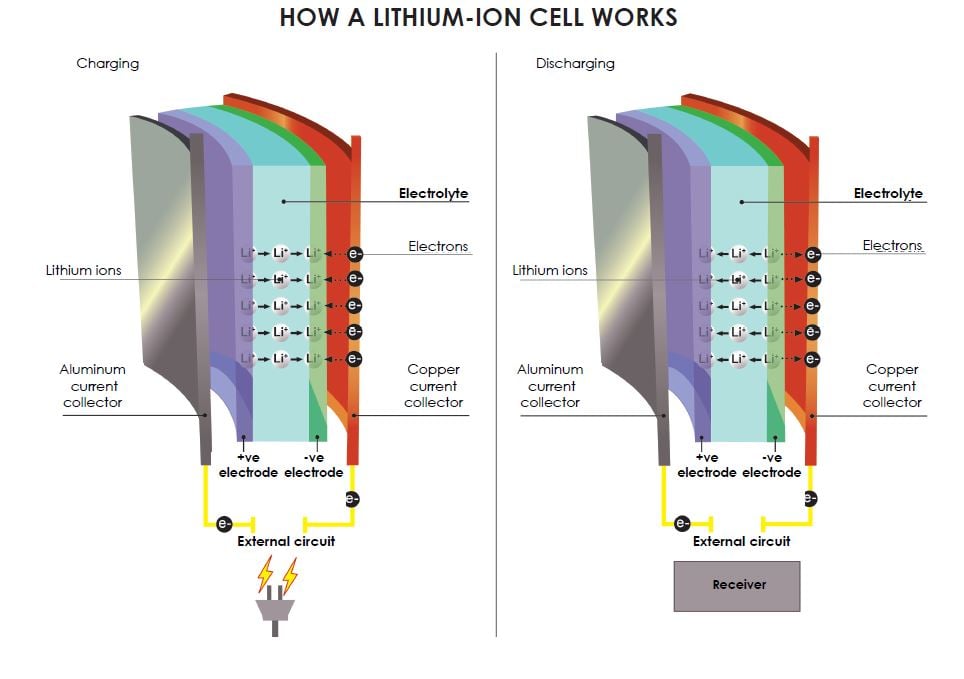
When the battery is being charged up, Li+ lithium ions leave the positive electrode (cathode) and are stored in the negative electrode (anode). When it is discharged to produce an electric current, the Li+ ions move in the opposite direction.
These cells, each with a voltage of a few volts, can be grouped together in varying numbers, depending on the capacity required to power a cell phone or car battery.
Advantages and Disadvantages
Lithium-ion batteries have a high , meaning that they can store three to four times more energy per unit mass than batteries using other technology. They are quick to recharge and can be used over and over again, with at least 500 discharge/charge cycles at 100%.
However, they are at risk of suddenly catching fire and releasing toxic gases due to the electrolyte overheating to above 100°C, known as thermal runaway. This has led to thousands of cell phones and tablets being recalled by manufacturers in recent years. In 2013, a battery in a Boeing 787 aircraft caught fire after landing.
Investigations have shown that overheating is most often caused by a short circuit brought on by incorrect assembly or impact. As a result, manufacturers are now required to follow rigorous processes and fit the lithium-ion batteries they make with an electronic battery management system (BMS), which turns the power off if it detects an anomaly.
In addition, manufacturers are looking into innovative technology that could help prevent overheating, such as solid electrolytes made of ultrathin polymer films.
New "gigafactories" in Europe
The decision to ban the sale of internal combustion engines after 2035 means that Europe must catch up in battery production. Several countries, in conjunction with vehicle manufacturers and startups, have launched projects to design new (lithium) battery models, with the challenge of them for future production.
"Gigafactories" dedicated to the production of batteries with a capacity of several gigawatt-hours (GWh) are being developed, with the long-term aim of taking over part of the market currently held by Asia (China, South Korea and Japan). In addition to the creation of these sites, the products themselves are the subject of innovations to equip the vehicles of tomorrow with more efficient batteries... and at lower cost.










