How Does a Photovoltaic Cell Work ?
5 min read
A is an electronic component that converts solar energy into electrical energy. This conversion is called the , which was discovered in 1839 by French physicist Edmond Becquerel1. It was not until the 1960s that photovoltaic cells found their first practical application in satellite technology. Solar panels, which are made up of PV cell modules, began arriving on rooftops at the end of the 1980s. Photovoltaic capacity has been growing steadily since the start of the 21st century, led by the construction of huge solar farms.

© Toru YAMANAKA / AFP - A Japanese researcher examines a new type of organic photovoltaic cell with photosensitive pigments.
How a Photovoltaic Cell Works
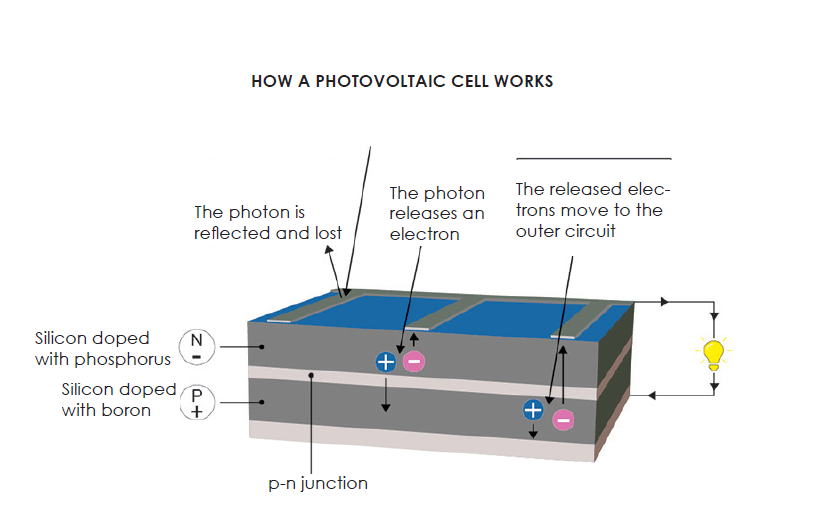
A is made of materials that absorb the light photons emitted by the sun and generate a flow of electrons. Photons are elementary particles that carry solar radiation at a speed of 300,000 kilometers per second. In the 1920s, Albert Einstein referred to them as “grains of light”. When the photons strike a semiconductor material like , they release the electrons from its atoms, leaving behind a vacant space. The stray electrons move around randomly looking for another “hole” to fill.
To produce an electric current, however, the electrons need to flow in the same direction. This is achieved using two types of silicon. The silicon layer that is exposed to the sun is doped with atoms of phosphorus, which has one more electron than silicon, while the other side is doped with atoms of , which has one less electron. The resulting sandwich works much like a battery: the layer that has surplus electrons becomes the negative terminal (n) and the side that has a deficit of electrons becomes the positive terminal (p). An electric field is created at the junction between the two layers.
When the electrons are excited by the photons, they are swept to the n-side by an electric field, while the holes drift to the p-side. The electrons and holes are directed to the electrical contacts applied to both sides before flowing to the external circuit in the form of electrical energy. This produces direct current. An anti-reflective coating is added to the top of the cell to minimize photon loss due to surface reflection. See the diagram below.
What is the efficiency of a photovoltaic cell?
Efficiency is the of electrical produced by the cell to the amount of sunlight it receives. To measure efficiency, the cells are combined into modules, which are in turn assembled into arrays. The resulting panels are then placed in front of a solar simulator that mimics ideal sunlight conditions: 1,000 watts (W) of light per cubic meter at an ambient temperature of 25°C. The electrical power produced by the system, or peak power, is a percentage of the incoming solar energy. If a panel measuring one square meter generates 200 W of electrical power, it has an efficiency of 20%. The maximum theoretical efficiency of a PV cell is around 33%. This is referred to as the Shockley-Queisser limit.
For example, if a 1 m2 panel produces an electrical power of 200 W per m2, which is often the level of a commercial panel today, its efficiency is 20%.
In real-life conditions, the amount of produced by a cell, known as the "productible", will depend not only on this efficiency but also on the region's average level of sunshine over a year. A panel with the same efficiency will produce three times more electricity in the Sahara desert than in the Paris region.
What is the output of a photovoltaic panel?
This is the ratio between the electrical power produced and the luminous power of the sun falling on the panel. It's around 20% for most panels on the market.
Different Types of Photovoltaic Cells
There are different types of PV cells. Their conversion efficiencies are being improved all the time.
Crystalline Silicon Cells
Silicon is extracted from silica. The latter has many forms, including quartz, which is found in large quantities in sand. Silicon cells account for more than 95% of the solar cell market. In commercial applications, their efficiency ranges from 16.5% to 22%, depending on the technology used.
In the cold processing method, the silicon is composed of many crystals and is called polycrystalline. The cells are easy to manufacture and can reach laboratory efficiency of over 22%. With treatment, silicon can be melted and converted into a large, single crystal structure and is called monocrystalline. It has a laboratory efficiency of up to 26.6%. The price of silicon cells has dropped in recent years, making PVs very competitive with other sources of electricity.
Thin-Film Cells
Instead of cutting silicon wafers of around 200 microns2, it is possible to semiconductor material in thin layers only a few microns thick on a substrate such as glass or plastic. Cadmium telluride or CIGS (copper / indium / gallium / selenium) can be used as semiconductors. Laboratory efficiency is very similar to silicon, but these cells have the advantage of being more flexible, lighter and less expensive.
Organic photovoltaic cells (OPV)
Organic solar cells that utilize organic molecules or polymers rather than semiconducting minerals are starting to be commercially applied. The cells continue to have a low conversion efficiency and a short lifetime but are potentially a low-cost alternative in terms of production. Another technology, dye-sensitized solar cells with photosensitive pigments, inspired by , is beginning to attract attention.
What is "organic" photovoltaics?
This technique uses organic chemistry molecules rather than mineral semiconductors such as silicon.
Perovskites
Earlier research on organic photovoltaics (OPV) led to the discovery of a new type of cell called perovskite, which uses hybrid organic-inorganic compounds as the active material. Perovskites have already reached laboratory efficiencies in line with those of other technologies (the record is 23.7%).
Although a lot of research still needs to be done before the cells can be mass produced (instability is a problem), perovskites have many advantages. In addition to being lightweight and flexible, their materials can be mixed with ink and applied to large surfaces. Furthermore, they are extremely cost effective to produce.
Technological Convergence
Scientists around the world are working on combining different PV technologies to create multi-junction cells. Studies are focusing on double "tandem" cells, combining silicon and thin films.
The efficiency of a cell with a single P-N junction cannot exceed a theoretical limit of around 33%, known as the "Shockley-Queisser limit". Cells with multiple P-N junctions, have a theoretical limit of over 50%. Already, every year, laboratory cell efficiency records reach 48%.
- His son, Henri Becquerel discovered the principles of in 1903, together with Pierre and Marie Curie.
- 1 micron = 1/1000th of a millimeter.